ANGIO-SEAL® (VIP Vascular Closure Device)
PRODUCT OVERVIEW

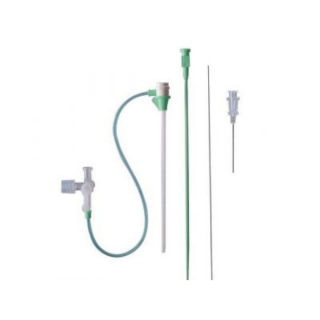
BIOABSORBABLE COMPONENTS
The device creates a mechanical seal by sandwiching the arteriotomy between a bioabsorbable anchor and collagen sponge, which dissolve in 60 to 90 days.
Three bioabsorbable components actively seal the arteriotomy:
- Anchor: Bioabsorbable co-polymer anchor placed against the inside of the vessel wall
- Collagen: Placed on top of the arteriotomy in the tissue tract
- Suture: Cinches the anchor and collagen together to form a secure seal.
LOCATE THE ARTERY
- Exchange the procedure sheath with the Angio-Seal locator system.
- Blood flow through the locator visually confirms proper sheath position in the artery.
SET THE ANCHOR
- Insert the Angio-Seal VIP device into the sheath until you hear a “click.”
- Gently pull back on the locking cap until you hear another “click.”
- The anchor is now locked in place and device is ready to be deployed.
SEAL THE PUNCTURE
- Gently pull back on the Angio-Seal VIP device until the suture has stopped spooling.
- Maintain upward tension on the device and gently advance the compaction tube until resistance is felt.
- Cut the suture and remove the device.
ANGIO-SEAL VIP PRODUCT CODES
| Part Number | French Size | Guidewire Diameter (in) |
| 610130 | 6Fr | .035 Wire |
| 610131 | 8Fr | .038 Wire |
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
- Nash JE, Evans DG. (1999). The Angio‐Seal hemostatic puncture closure device. Concept and experimental results. Herz, 24(8), 597‐606.
- Per Instructions For Use ASIN0004 revision 2018-09-01.
Indications:
The Angio-Seal Vascular Closure Device product family, including the VIP and Evolution platforms, is indicated for use in closing and reducing time to hemostasis of the femoral arterial puncture site in patients who have undergone diagnostic angiography procedures or interventional procedures using an 8 French or smaller procedural sheath for the 8 F Angio-Seal device and a 6 French or smaller procedural sheath for the 6 F Angio-Seal device. The Angio-Seal VIP and Evolution platform devices are also indicated for use to allow patients who have undergone diagnostic angiography to safely ambulate as soon as possible after sheath removal and device placement, as well as to allow patients who have undergone an interventional procedure to safely ambulate after sheath removal and device placement.
Important Safety Information:
Possible adverse events for vascular closure devices include, but are not limited to: bleeding or hematoma, AV fistula or pseudoaneurysm, infection, allergic reaction, foreign body reaction, inflammation or edema. This device should only be used by a licensed physician (or other health care professional authorized by or under the direction of such physician) possessing adequate instruction in the use of the device, e.g., participation in an Angio-Seal physician instruction program or equivalent.
ANGIO-SEAL® (VIP Vascular Closure Device)
Related products
-
RADIFOCUS INTRODUCER SHEATH
Radifocus™ Introducer II Standard Kit A
Introducer Sheath (FEMORAL)
Kit of vascular introducer with hemostatic valve with excellent blood tightness,
easy and non traumatic vessel insertion, patented valve design for a unique
features package.- Total Integrated Fit (TIF) tip tapering: tapering design at the tip of the sheath and dilator to facilitate smooth penetration
- Cross-cut hemostasis valve to avoid blood reflux and air aspiration
- Thin radiopaque sheath with anti-kinking sleeve for easy catheter handling
- Snap-on/click-off dilator lock prevents dilator back-out during insertion and allows one-hand unlocking
- Wide variety of kit variations providing all elements for quick vessel access: 4-11 Fr sheaths, 5-16 cm lengths, Surf lash needle
General specifications
Sheath length 10 cm Mini guidewire Plastic Straight and Angled
0.025″ (0.64 mm) for 4 Fr, straight mini guidewire type; 0.035″ (0.89 mm) for all others
45 cmEntry needle Plastic IV Catheter – 18G x 2 ½” (1.2 x 64 mm),
except for 4 Fr with 45 cm straight mini guidewire: 20G x 2″ (0.9 x 51 mm)
2.5 ml syringe is includedGuidewire compatibility 0.025″ (0.64 mm) for 4 Fr, straight mini guidewire type
0.035″ (0.89 mm) for all othersPackaging Tray A Kit content Sheath, dilator, plastic mini guidewire and plastic IV catheter (entry needle) Item specifications
Inner diameter Mini guidewire type 45 cm angled 45 cm straight 4 Fr RS+A40K10AQ RS+A40G10SQ 4 Fr RS*A40G10SQ RS*A40G05SQ 4 Fr RS*A40K10SQ RS*A40G07SQ 5 Fr RS*A50K10AQ RS*A50G07SQ 5 Fr RS+A50K10AQ RS+A50K10SQ 6 Fr RS+A60K10AQ RS+A60K10SQ 6 Fr RS*A60K10AQ RS*A60G07SQ 6 Fr — RS*A60G16SQZ 6 Fr — RS*A60K10SQ 6 Fr — RS+A50K10SQ 7 Fr — RS+A70K10SQ 7 Fr — RS*A70G07SQ 7 Fr — RS*A70K10SQ 8 Fr — RS+A80K10SQ 8 Fr — RS*A80K10SQ 9 Fr — RS*A90K10SQ 10 Fr — RS*A10K10SQ 11 Fr — RS*A11K10SQ A Kit includes: introducer sheath, dilator, mini Guide wire, entry venous catheter, 2.5 cc syringe
B Kit includes: introducer sheath, dilator, mini Guide wire
C Kit includes: introducer sheath, dilator
R Kit includes: introducer sheath, dilator, mini Guide wire, 18 G x 2 3/4” / 1.2 x 70 mm entry metallic needle
Other code numbers are available on special demand. For any further information, please contact your Terumo local representative
Units per box: 5 for kits in tray and 10 for kits in pouch. -
Launcher- 6f Guiding Catheter’s
EBU@ (Extra Backup)
LA6EBU30 (EBU 3.0)
LA6EBU35 (EBU 3.5)
LA6EBU375 (EBU 3.75)
LA6EBU40 (EBU 4.0)
LA6EBU45 (EBU 4.5)
LA6EBU50 (EBU 5.0)
JL@ (Judkin Left )
LA6JL30 (JL 3.0)
LA6JL35 (JL 3.5)
LA6JL40 (JL 4.0)
LA6JL45 (JL 4.5)
LA6JL50 (JL 5.0)
LA6JL60 (JL 6.0)
JR@ (Judkin Right )
LA6JR30 (JR 3.0)
LA6JR35 (JR 3.5)
LA6JR40 (JR 4.0)
LA6JR45 (JR 4.5)
LA6JR50 (JR 5.0)
LA6JR60 (JR 6.0)
AL@ (Amplatz Left )
LA6AL75 (AL .75)
LA6AL10 (AL 1.0)
LA6AL15 (AL 1.5)
LA6AL20 (AL 2.0)
LA6AL25 (AL 2.5)
LA6AL30 (AL 3.0)
LA6AL40 (AL 4.0)
SAL@ (Short Amplatz Left)
LA6SAL75 (SAL . 75)
LA6SAL10 (SAL 1.0)
LA6SAL15 (SAL 1.5)
LA6SAL20 (SAL 2.0)
LA6SAL25 (SAL 2.5)
LA6SAL30 (SAL 3.0)
LA6SAL40 (SAL 4.0)
AR@ (Amplatz Right)
LA6AR10 (AR 1.0)
LA6AR20 (AR 2.0)
LA6ALR12 (ALR 1.2)
ECR Curves@ (Backup Support Right)
LA6ECR35 (ECR 3.5)
LA6ECR40 (ECR 4.0)
LA6ECR45 (ECR 4.5)
LA6RBU35 (RBU 3.5)
LA6RBU40 (RBU 4.0)
SCR@ (Shepherd’s Crook Right)
LA6SCR35 (SCR 3.5)
LA6SCR40 (SCR 4.0)
LA6SCR50 (SCR 5.0)
SAR@ (Short Amplatz Right)
LA6SAR10 (SAR 1.0)
LA6SAR20 (SAR 2.0)
Multipurpose@
LA6MB1 (MB 1)
LA6MB2 (MB 2)
LA6HSREL (Hockey Stick)
LA6HSI (Hockey I)
LA6HSII (Hockey II)
LA6HSIII (Hockey III)
Bypass Crafts@
LA6LCB (LCB)
LA6RCB (RCB)
LA6RCB (RCB III)
LA6RCB (RCB 90cm)
LA6IMA (IMA)
LA6IMA (IMA 90cm)
-
NC EUPHORA NONCOMPLIANT BALLOON CATHETER
NC Euphora™ noncompliant balloon
NC Euphora™ noncompliant balloon dilatation catheter combines Euphora technology with controlled, high-pressure performance.
Balloon Dilatation Catheter
OVERVIEW
From Collaboration to Innovation
When it comes to controlled, high-pressure performance, the NC Euphora™ noncompliant balloon dilatation catheter has what you’re looking for:
- High RBP up to 20 atm
- Low-growth profile1
- Superior deliverability1
In fact, we worked with nearly 2,000 interventional cardiologists and cath lab professionals to develop it — so you can choose it with confidence for your patients.
PRODUCT DETAILS
Looking for NC Euphora Noncompliant Performance?
ORDERING INFORMATION
BALLOON LENGTHS 6, 8, AND 12 MM
Balloon
Diameter
(mm)Balloon Length (mm) 6 8 12 2.00 NCEUP2006X NCEUP2008X NCEUP2012X 2.25 NCEUP22506X NCEUP22508X NCEUP22512X 2.50 NCEUP2506X NCEUP2508X NCEUP2512X 2.75 NCEUP27506X NCEUP27508X NCEUP27512X 3.00 NCEUP3006X NCEUP3008X NCEUP3012X 3.25 NCEUP32506X NCEUP32508X NCEUP32512X 3.50 NCEUP3506X NCEUP3508X NCEUP3512X 3.75 NCEUP37506X NCEUP37508X NCEUP37512X 4.00 NCEUP4006X NCEUP4008X NCEUP4012X 4.50 — NCEUP4508X NCEUP4512X 5.00 — NCEUP5008X NCEUP5012X BALLOON LENGTHS 15, 20, AND 27 MM
Balloon
Diameter
(mm)Balloon Length (mm) 15 20 27 2.00 NCEUP2015X NCEUP2020X — 2.25 NCEUP22515X NCEUP22520X — 2.50 NCEUP2515X NCEUP2520X NCEUP2527X 2.75 NCEUP27515X NCEUP27520X NCEUP27527X 3.00 NCEUP3015X NCEUP3020X NCEUP3027X 3.25 NCEUP32515X NCEUP32520X NCEUP32527X 3.50 NCEUP3515X NCEUP3520X NCEUP3527X 3.75 NCEUP37515X NCEUP37520X NCEUP37527X 4.00 NCEUP4015X NCEUP4020X NCEUP4027X 4.50 NCEUP4515X NCEUP4520X — 5.00 NCEUP5015X — — -
RESOLUTE INTEGRITY STENT
Resolute Onyx DES
for coronary artery disease
Resolute Onyx™ is a drug-eluting stent (DES) that’s different by design, optimised for complex PCI, and proven safe and effective in real-world, high bleeding risk patients on 1-month dual antiplatelet therapy (DAPT).1
Resolute Onyx DES is different by design to address your DES needs and a wide range of patient anatomies. It features:
- Best-in-class deliver ability
- Enhanced visibility with thinner struts
- Smooth side branch access
- Fast healing
- The broadest diameter range
Best-in-class deliverability
Single-wire design
Resolute Onyx DES is made from a single wire, which gives it a fluid range of motion and provides the flexibility needed for best-in-class deliverability.2

Sinusoid-formed wire

Helical wrap

Laser-fused
Competitive comparison
Resolute Onyx DES is more deliverable than laser-cut SYNERGY™* XD and XIENCE Skypoint™*, which are stiffer.
Deliverability comparison — 3.0 mm DES2
(Lower is better)

Enhanced visibility with thinner struts
Platinum-iridium core within Resolute Onyx DES increases visibility for accurate stent placement without compromising strut thickness.2Average visibility comparison2
(Higher is better)

- Cobalt alloy shell
- Platinum iridium core

Resolute Onyx cobalt alloy shell and platinum-iridum core
Smooth side branch access
Resolute Onyx DES has round struts to create a smooth passage when accessing the side branch and lower the propensity to catch during bifurcation procedures.2
Strut comparison
Rounded strut cross-section
- Resolute Onyx DES

Square strut cross-section
- SYNERGY™* DES
- XIENCE™* DES

Broadest diameter range
- Complex PCI requires a broad DES size matrix to match a wide range of patient anatomies.
- Resolute Onyx is the only DES with diameters ranging from 2.0 mm to 5.0 mm to treat a broad range of coronary vessel sizes.
Diameter (mm) Stent Length (mm) Maximum Expansion
Capabilities (MSID†) (mm)2.00 8 12 15 18 22 26 30 — — 3.50‡ 2.25 8 12 15 18 22 26 30 34 38 3.50‡ 2.50 8 12 15 18 22 26 30 34 38 3.50‡ 2.75 8 12 15 18 22 26 30 34 38 4.00‡ 3.00 8 12 15 18 22 26 30 34 38 4.00‡ 3.50 8 12 15 18 22 26 30 34 38 5.00‡ 4.00 8 12 15 18 22 26 30 34 38 5.00‡ 4.50 — 12 15 18 22 26 30 — — 6.00‡ 5.00 — 12 15 18 22 26 30 — — 6.00‡ PRODUCT DETAILS
2.00 RONYX20008X RONYX20012X RONYX20015X RONYX20018X RONYX20022X RONYX20026X RONYX20030X N/A N/A 2.25 RONYX22508X RONYX22512X RONYX22515X RONYX22518X RONYX22522X RONYX22526X RONYX22530X RONYX22534X RONYX22538X 2.50 RONYX25008X RONYX25012X RONYX25015X RONYX25018X RONYX25022X RONYX25026X RONYX25030X RONYX25034X RONYX25038X 2.75 RONYX27508X RONYX27512X RONYX27515X RONYX27518X RONYX27522X RONYX27526X RONYX27530X RONYX27534X RONYX27538X 3.00 RONYX30008X RONYX30012X RONYX30015X RONYX30018X RONYX30022X RONYX30026X RONYX30030X RONYX30034X RONYX30038X 3.50 RONYX35008X RONYX35012X RONYX35015X RONYX35015X RONYX35022X RONYX35026X RONYX35030X RONYX35034X RONYX35038X 4.00 RONYX40008X RONYX40012X RONYX40015X RONYX40018X RONYX40022X RONYX40026X RONYX40030X RONYX40034X RONYX40038X 4.50 N/A RONYX45012X RONYX45015X RONYX45018X RONYX45022X RONYX45026X RONYX45030X N/A N/A 5.00 N/A RONYX50012X RONYX50015X RONYX50018X RONYX50022X RONYX50026X RONYX5
















Reviews
There are no reviews yet.