ANGIO-SEAL® (VIP Vascular Closure Device)
PRODUCT OVERVIEW

BIOABSORBABLE COMPONENTS
The device creates a mechanical seal by sandwiching the arteriotomy between a bioabsorbable anchor and collagen sponge, which dissolve in 60 to 90 days.
Three bioabsorbable components actively seal the arteriotomy:
- Anchor: Bioabsorbable co-polymer anchor placed against the inside of the vessel wall
- Collagen: Placed on top of the arteriotomy in the tissue tract
- Suture: Cinches the anchor and collagen together to form a secure seal.
LOCATE THE ARTERY
- Exchange the procedure sheath with the Angio-Seal locator system.
- Blood flow through the locator visually confirms proper sheath position in the artery.
SET THE ANCHOR
- Insert the Angio-Seal VIP device into the sheath until you hear a “click.”
- Gently pull back on the locking cap until you hear another “click.”
- The anchor is now locked in place and device is ready to be deployed.
SEAL THE PUNCTURE
- Gently pull back on the Angio-Seal VIP device until the suture has stopped spooling.
- Maintain upward tension on the device and gently advance the compaction tube until resistance is felt.
- Cut the suture and remove the device.
ANGIO-SEAL VIP PRODUCT CODES
| Part Number | French Size | Guidewire Diameter (in) |
| 610130 | 6Fr | .035 Wire |
| 610131 | 8Fr | .038 Wire |
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
- Nash JE, Evans DG. (1999). The Angio‐Seal hemostatic puncture closure device. Concept and experimental results. Herz, 24(8), 597‐606.
- Per Instructions For Use ASIN0004 revision 2018-09-01.
Indications:
The Angio-Seal Vascular Closure Device product family, including the VIP and Evolution platforms, is indicated for use in closing and reducing time to hemostasis of the femoral arterial puncture site in patients who have undergone diagnostic angiography procedures or interventional procedures using an 8 French or smaller procedural sheath for the 8 F Angio-Seal device and a 6 French or smaller procedural sheath for the 6 F Angio-Seal device. The Angio-Seal VIP and Evolution platform devices are also indicated for use to allow patients who have undergone diagnostic angiography to safely ambulate as soon as possible after sheath removal and device placement, as well as to allow patients who have undergone an interventional procedure to safely ambulate after sheath removal and device placement.
Important Safety Information:
Possible adverse events for vascular closure devices include, but are not limited to: bleeding or hematoma, AV fistula or pseudoaneurysm, infection, allergic reaction, foreign body reaction, inflammation or edema. This device should only be used by a licensed physician (or other health care professional authorized by or under the direction of such physician) possessing adequate instruction in the use of the device, e.g., participation in an Angio-Seal physician instruction program or equivalent.
ANGIO-SEAL® (VIP Vascular Closure Device)
Related products
-
GUIDING CATHETER EKARI
Product Overview
IMPROVED BACK UP SUPPORT*1
Catheter designed to use contralateral wall resulting in enhanced back-up support.
UNIVERSAL SHAPE (IKARI LEFT) *2
Offer capability of accessing the right and left coronary arteries, potentially avoiding catheter exchanges.
DESIGN FOR SAFETY
Soft tip to reduce damage to the vessel wall

*1: Ikari Y, Nagaoka M, Kim JY, Merino Y, Tanabe T. The physics of guiding catheters for the left coronary artery in transfemoral and trans-radial interventions. J Invasive Cardio. 2005 Dec; 17(12): 636-641.*2: Youssef AA, Hsieh YK, Cheng CI, We CJ. A single trans-radial guiding catheter for right and left coronary angiography and intervention. Euro intervention 2007; 3: 475-481.
Patients who have had cardiac catheterization or coronary angioplasty often are required to stay in bed with restricted movement for three to 24 hours afterward to prevent bleeding from the femoral artery catheter insertion site.
Features and Benefits
Improved back up support in comparison to Terumo standard femoral guiding catheter shapes*1

*1: Based on comparative studies made among Terumo Guiding Catheters*2: Ekari Y, Nagaoka M, Kim JY, Merino Y, Tanabe T. The physics of guiding catheters for the left coronary artery in transfemoral and trans-radial interventions. J Invasive Cardio. 2005 Dec; 17(12): 636-641.*3: Youssef AA, Hsieh YK, Cheng CI, We CJ. A single trans-radial guiding catheter for right and left coronary angiography and intervention. Euro intervention 2007; 3: 475-481.
Guide catheters are required for all coronary interventions to provide access to the coronary ostium and support equipment delivery. The ideal guide provides stability for device advancement through the coronary anatomy, while minimizing vessel trauma and allowing for vessel opacification.
The catheter is guided to the narrowed artery. Then, a smaller balloon catheter is inserted through the flexible catheter and inflated at the narrowed area to open it. Often, the doctor will also place a mesh coil called a stent at the narrowed part to help keep the artery open.
-
FINECROSS Microcatheter
FINECROSS® MG Coronary Micro-Guide Catheter
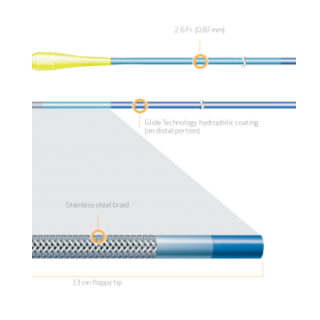
Product Code Catheter Length Distal Hydrophilic Coating Distal Outer Diameter Proximal Outer Diameter Distal Inner Diameter Proximal Inner Diameter Recommended Guidewire Size 35-1430 130cm 70cm 1.8Fr. (0.60mm) 2.6 Fr. (0.87mm) 0.018″ (0.45mm) 0.021″ (0.55mm) 0.014″ (0.36mm) 35-1450 150cm 90cm 1.8Fr. (0.60mm) 2.6 Fr. (0.87mm) 0.018″ (0.45mm) 0.021″ (0.55mm) 0.014″ (0.36mm) PRODUCT OVERVIEW
Coronary microguide catheter for integration of optimal guidewire support, superior trackability and crossability.1,2

TAPERED STAINLESS STEEL BRAID CONSTRUCTION FOR OPTIMAL GUIDEWIRE SUPPORT
FINECROSS MG stainless steel braid construction is designed to provide strength, responsiveness and support for improved pushability to access and cross complex lesions.
13cm FLOPPY DISTAL SEGMENT FOR SUPERIOR TRACKABILITY1
The distal 13 cm is ultra flexible for improved trackability around tight bends and tortuous anatomy.The floppy distal segment is designed to be atraumatic and provide an optimal balance between trackability and safety while navigating through the tortuous anatomy.1
TAPERED OUTER DIAMETER FOR SUPERIOR CROSSABILITY1,2
The outer diameter of the stainless steel shaft tapers from a proximal 2.6Fr. to a distal 1.8Fr. designed for improved crossability and guidewire handling.1,2
DOCUMENTS
-
EUPHORA SEMICOMPLIANT BALLOON CATHETER
Euphora™ semicompliant balloon
Balloon Dilatation Catheter
Euphora™ semicompliant balloon dilatation catheter complements your expertise and provides uncompromising performance in the moment you need it most.
OVERVIEW
Advancing Innovation through Partnership
To develop the Euphora™ low-profile semicompliant balloon dilatation catheter, we partnered with nearly 2,000 interventional cardiologists and cath lab professionals. The result is:
- Superior deliverability1
- Superb Kissing Balloons Technique (KBT) performance2
- The lowest crossing profile compared to leading competitors3
PRODUCT DETAILS
What Makes the Euphora Balloon an Excellent Choice?
ORDERING INFORMATION
BALLOON LENGTHS 6, 10, 12, AND 15 MM
1.50 EUP1506X EUP1510X EUP1512X EUP1515X 2.00 EUP2006X EUP2010X EUP2012X EUP2015X 2.25 EUP22506X EUP22510X EUP22512X EUP22515X 2.50 EUP2506X EUP2510X EUP2512X EUP2515X 2.75 EUP27506X EUP27510X EUP27512X EUP27515X 3.00 EUP3006X EUP3010X EUP3012X EUP3015X 3.25 EUP32506X EUP32510X EUP32512X EUP32515X 3.50 EUP3506X EUP3510X EUP3512X EUP3515X 3.75 EUP37506X EUP37510X EUP37512X EUP37515X 4.00 EUP4006X EUP4010X EUP4012X EUP4015X BALLOON LENGTHS 20, 25, AND 30 MM
1.50 EUP1520X — — 2.00 EUP2020X EUP2025X EUP2030X 2.25 EUP22520X EUP22525X — 2.50 EUP2520X EUP2525X EUP2530X 2.75 EUP27520X EUP27525X — 3.00 EUP3020X EUP3025X EUP3030X 3.25 EUP32520X EUP32525X — 3.50 EUP3520X EUP3525X EUP3530X 3.75 EUP37520X EUP37525X — 4.00 EUP4020X EUP4025X EUP4030X -
SPRINTER LEGEND PTCA BALLOON
SPRINTER LEGEND 1.25 BALLOON CATHETER
Super Crosser Semi compliant Balloon Dilatation Catheter (RX/OTW)
Sprinter™ Legend™ 1.25 SuperCrosser semicompliant balloon dilatation catheter delivers exceptional crossability to treat today’s challenging lesions.
With innovative crossing technologies and 57 sizes, the Sprinter™ Legend™ RX semicompliant balloon dilatation catheter provides you with the power to cross.
OVERVIEW
During difficult cases, reach for a slim Sprinter™ Legend™ 1.25 mm Super Crosser semi compliant coronary balloon catheter featuring:
- Award-winning* Zerofold technology — no wrapped material and no balloon shoulders
- Low 0.5 mm (0.020 in) crossing profile
- OTW and RX platforms
ORDERING INFORMATION
BALLOON LENGTHS 6, 10, 12, AND 15 MM
1.25 SPL12506WL SPL12510WL SPL12515WL SPL12520WL Sprinter Legend RX Semi compliant
Balloon Dilatation Catheter
OVERVIEW
Performance You Can Trust
Sprinter™ Legend™ semicompliant balloons help you cross, open, and treat lesions during challenging percutaneous coronary interventions (PCIs), and feature:
- Some of the industry’s lowest crossing profiles1
- Innovative crossing technology
- A broad range of sizes
ORDERING INFORMATION
BALLOON LENGTHS 6, 10, 12, AND 15 MM
Balloon Diameter (mm)
1.25 SPL12506X SPL12510X SPL12512X SPL12515X 1.50 SPL15006X SPL15010X SPL15012X SPL15015X 2.00 SPL20006X SPL20010X SPL20012X SPL20015X 2.25 SPL22506X SPL22510X SPL22512X SPL22515X 2.50 SPL25006X SPL25010X SPL25012X SPL25015X 2.75 SPL27506X — SPL27512X SPL27515X 3.00 SPL30006X SPL30010X SPL30012X SPL30015X 3.25 — — SPL32512X SPL32515X 3.50 SPL35006X SPL35010X SPL35012X SPL35015X 3.75 — — SPL37512X SPL37515X 4.00 SPL40006X SPL40010X SPL40012X SPL40015X BALLOON LENGTHS 20, 25, AND 30 MM
Balloon Diameter (mm)
1.25 SPL12520X — — 1.50 SPL15020X — — 2.00 SPL20020X SPL20025X SPL20030X 2.25 SPL22520X SPL22525X — 2.50 SPL25020X SPL25025X SPL25030X 2.75 SPL27520X SPL27525X — 3.00 SPL30020X SPL30025X SPL30030X 3.25 SPL32520X — — 3.50 SPL35020X SPL35025X SPL35030X 3.75 SPL37520X — — 4.00 SPL40020X SPL40025X SPL40030X







Reviews
There are no reviews yet.